How do we know how old the last ice age was? How do we know how old our oldest ancestors are? How do we know how old the Earth is? Isotopes is how and this is my guide to understanding them and how they are used in dating.
What is an isotope?
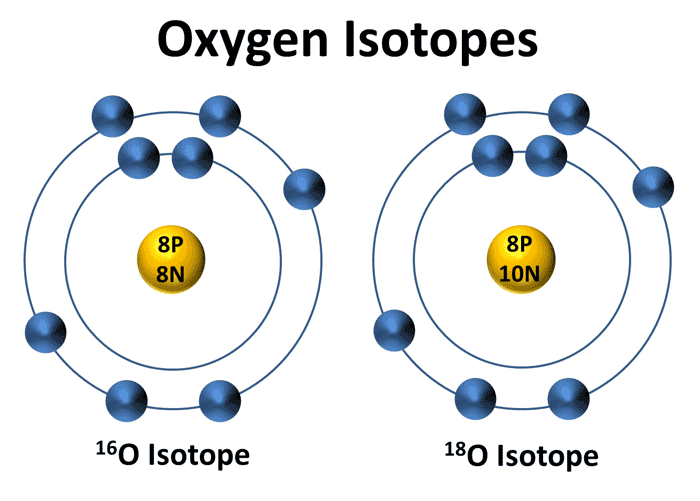
An element is defined by its protons, neutrons and electrons. Isotopes are elements that have differing number of neutrons to protons, affecting elemental mass but keeping chemical properties similar. Physical properties do change isotope to isotope affecting mass, melting, boiling and freezing points as well as density. These changes in physical properties are what scientists use to track and determine age and environment such as the decay of Carbon-14 or the ratio between Oxygen-16 and Oxygen-18 used in climate reconstruction.
How are oxygen isotopes used to reconstruct climate?
So now that we understand what isotopes are and why they matter to scientists, I can now further elaborate on the methodological approach as to why scientists care so much. The ratio between Oxygen-16 and Oxygen-18 are key in determining what the climate was like at any point in time. Oxygen-16 is lighter (less neutrons) than Oxygen-18 (more neutrons). The lighter isotope thus allows for evaporation to occur easier from water bodies meaning a warmer environment.
How do we get this isotope ratio?
Scientists gain this data from ice cores, tree rings and marine shells. All of these 'proxies' store oxygen isotopes in different ways and in different volumes/ratios, giving an idea of the climate. Marine shells give an idea of water Oxygen-16/Oxygen-18 ratios. In water, if Oxygen-18 is abundant, it can be deemed to be a warmer climate due to less Oxygen-16 (large evaporation), however, if this ratio is closer together, the climate is likely cooler. The same is true for freezing ice, Oxygen-16, because it is lighter, freezes easier than Oxygen-18. This ratio can be determined as to whether the climate was freezing or not due to Oxygen-16 abundance.
Age and History
So now you're thinking: "Now I know what an isotope is and how they are used to determine climate, but how do we know how old they are?" GREAT question anonymous reader. This can be done through a variety of ways and rules used by scientists. Tree rings are a good example, the thicker rings display ideal environments for growth whereas thinner ones the converse and the location of these is important. Rings on the outside are closer to present day whereas rings closer to the interior are older. This is called dendrochronology, radiocarbon dating techniques (Carbon-14 decay) is used to determine age and width climate. Another rule in science is the law of superposition, simply, the deeper something is, the older it is. Marine shells buried further and further down in water sediment allow scientists to determine the age similar to tree rings.What now?
You now know: What an isotope is and why scientists use them to reconstruct climate with time and detail. Now it just comes down to putting this information into practice analysing long records of different climate proxies (tree rings, marine shells, ice cores), concluding climatic conditions and concluding a timeline of these events. These continuing our understanding of the Earth and its History and possible hypotheses for future millennia.



Comments
Post a Comment